Macular Degeneration and Stem Cells
Macular Degeneration Research Updates
Macular degeneration and stem cells are the focus of research by several biotech companies in the United States and world wide that seek to use regenerative medicine to treat many different retinal conditions.
There are several different ways to obtain, develop and utilize stem cells that don't require cells coming from an embryo. These cells are called adult (non-embryonic) stem cells.
This macular degeneration research involves regenerating or replacing the cells that are found in a
layer of tissue that lies under the retina.
There are three layers of tissue that support the retina - the choroid, Bruch's membrane and the retinal pigment epithelium.
Autologous Stem Cell Transplant
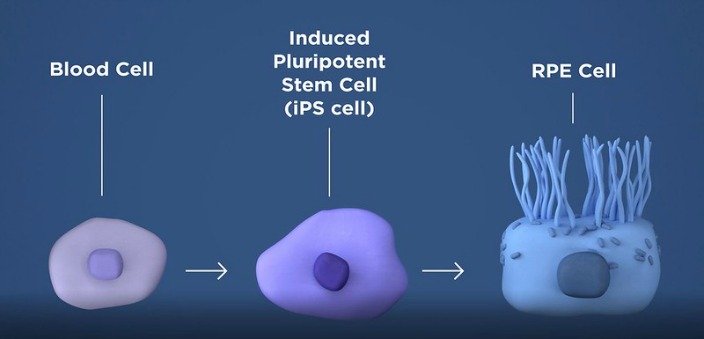
Autologous stem cells are cells that are taken from the patient's skin or blood and then given back to the patient via different transplant procedures. Stem cells can be produced by "winding cells backwards." A
patient's own skin or blood cells can be reprogrammed to become what is
called induced Pluripotent Stem cells (iPSC).
Induced Stem Cells from Patient's Blood for Advanced Dry AMD
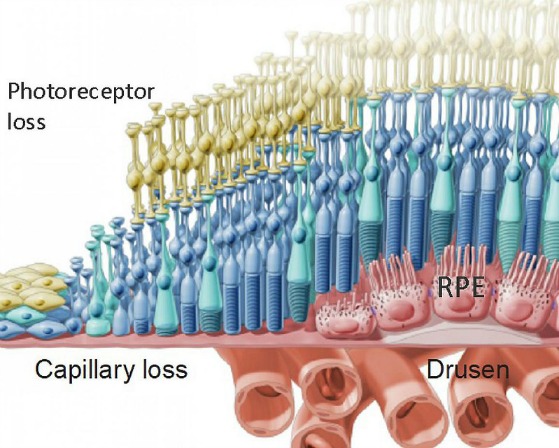
The loss of cells in the Retinal Pigment Epithelium (RPE), a layer of the retina that helps to nourish the macula contributes to age related macular degeneration and loss of vision.
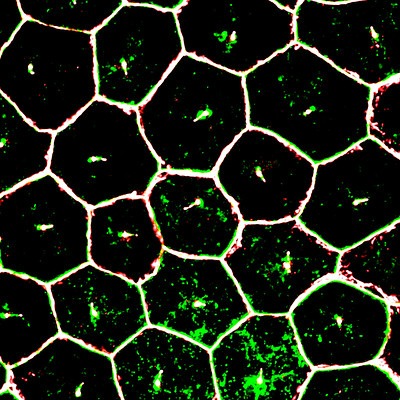
Image from the NIH shows mature iPSC (induced pluripotent stem cell) derived retinal pigment epithelium cells (RPE), magnified by super resolution confocal microscopy.
The National Eye Institute announced on December 16, 2019 a new study using a patient's own blood cells to program adult stem cells for the treatment of advanced dry macular degeneration called geographic atrophy.
Cells taken from the patient's blood will be converted to what are called induced pluripotent stem cells (iPs).
Once converted to iPs cells, these cells have the potential to become any type of cell. In this Phase 1 clinical trial the iPs cells will be programmed to be come retinal pigment epithelial (RPE) cells. The RPE is responsible for nourishing and supporting the photoreceptor cells. When the RPE cells degenerate the photoreceptor cells then follow which leads to the many different vision losses associated with macular degeneration
The iPSC cells that are now programmed to be RPE cells are grown in sheets that are one cell thick. The patch of cells is implanted between the RPE and photoreceptor cells with the intention they they will take on the role of nourishing the photoreceptor cells.
"Five participants will undergo RPE transplantation in one eye. Eligible eyes will have GA, best-corrected visual acuity (BCVA) between 20/100 and 20/500, and a fellow eye that has same or better BCVA. If the National Eye Institute (NEI) Data and Safety Monitoring Committee (DSMC) gives clearance to proceed based on review of data from the first cohort, a second cohort of up to seven additional participants with GA, BCVA between 20/80 and 20/500 in the eye being considered for RPE transplantation, and same or better visual acuity in the other eye may undergo the procedure to gather additional safety and potential efficacy data useful for planning future studies. Up to 20 participants may be enrolled to allow for screening failures or for participants withdrawing from the study prior to RPE transplantation." The study is seeking to generate and then transplant a layer of healthy RPE cells to prevent vision loss.
On August 31, 2022 the National Institutes of Health announced that the surgical team of eye specialists from Wilmer Eye Institute of Johns Hopkins School of Medicine successfully implanted a patch of tissue made from patient cells. This is the first time in the United States that patient derived induced pluripotent stem cells were used. The National Institutes of Health in their news release from August 31, 2022 explained that ..."In the NIH lab the patient's blood cells were converted to iPs cells, which can become almost any type of cell in the body. In this case, they were programmed to become retinal pigment epithelial (RPE) cells, the type of cell that degenerates in the advanced forms of dry AMD."
Autologous Stem Cells from Bone Marrow
The Ophthalmology Times article of June 15, 2015, Bone marrow cells show promise in degenerative, ischemic retinal diseases, states that "Intravitreal injection of autologous CD34+ stem cells from bone marrow may be both feasible and well tolerated in eyes with degenerative or ischemic retina diseases, said Susanna S. Park, MD, PhD," professor, Department of Ophthalmology and Vision Science, University of California-Davis Eye Center, Sacramento.
Patients who were diagnosed with retinal vascular occlusion, dry AMD or retinitis pigmentosa received an intravitreal injection of autologous CD34+bone marrow cells in a pilot study. The study's aim is "to evaluate both the safety and feasibility of intravitreal autologous CD34+ bone marrow cells as a potential therapy."
Clinical Trial of Autologous Intravitreal Bone-marrow CD34+ Stem Cells for Retinopathy
Research involving macular degeneration and stem cells continues to evolve and progress from lab research to early phase clinical trials.
Go from Macular Degeneration and Stem Cells to Dry AMD Clinical Trials
Go from Macular Degeneration and Stem Cells to WebRN Macular Degeneration Home
√ Prevention of Macular Degeneration?
√ Tips for Daily Living?
√ Food Suggestions for a Macular Degeneration Diet?
√ Ideas on Visual Aids to Maximize your Sight?
If you said "yes" to any of the above, sign up for the monthly Macular Degeneration News.