Laser Therapy for Macular Degeneration
Laser therapy for macular degeneration has been around for many years, but the way it is given and the type of technology used has changed in many ways to benefit the patient.
Original Hot Laser
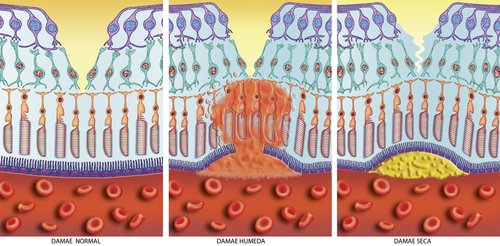
The original laser photocoagulation used a hot laser that sealed leaky blood vessels but also burned some of the retinal tissue. If the leak was in the macular area - the laser left a permanent scar that left the patient with some vision loss. It is still used today for clumps of blood vessels that are not under the macula.
Photodynamic Therapy
Recognizing that while a hot laser did indeed help to control leaking
blood vessels, a better option was needed for vessels growing under the
macula.
This two step procedure involves first the injection of a drug, Verteporfin into the arm via an IV.
Once the drug reaches the eye a cold laser is used to activate the drug and destroy the choroidal neovascularization.
This laser therapy for macular degeneration became less common once the new anti-VEGF intra-ocular injections became available.
Low Level Laser Therapy for Dry AMD
LumiThera, a medical device company, was awarded the "Most Innovative and Promising Medtech and E-Health Company" during Biovison 2017 in Lyon, France.
"We are delighted that Biovision has recognized the scientific, clinical and product development progress LumiThera made over the last year in advancing towards the commercial introduction of our innovative technology for treating dry age related macular degeneration (AMD), one of the leading causes of legal blindness in the world," said Clark Tedford, Ph.D., Chief Executive Officer. "We are honored to have received this second award as we prepare to bring this important therapy to the millions of patients suffering from dry AMD who have no other treatment alternatives."
Unlike hot laser therapy that destroys tissue photobiomodulation (PBM) uses low level light therapy to cause a photochemical reaction. “It’s been used for many years as an alternative to traditional anti-inflammatory drug medication. The technology has an outstanding safety record,” says Clark Tedford, Ph.D and one of the co-founders of LumiThera.
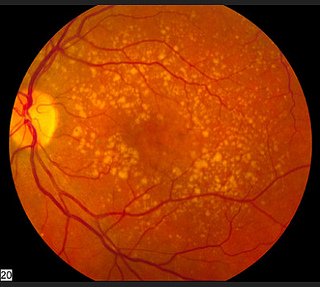
The conclusion of a 2012 small ( 18 eyes, 9 patients) pilot study done in Fort Lauderdale, Florida stated that "PBM proves to be beneficial for improvement of vision and contrast sensitivity and a safe treatment for dry AMD in this pilot study." Similar positive vision changes were reported In the article Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age‐related macular degeneration, published in Acta Ophthalmologica June 2017 with improved contrast sensitivity and improvement in mean Best Corrected Visual Acuity of 5.90 letters. Macular drusen volume and thickness were reduced as well.
LumiThera announced CE Mark Certification for the European Union for the treatment of dry AMD on June 22, 2018 using the LT-300 Light Delivery System. To obtain the “CE” (Conformite Européenne) mark or certification, the European Commission explains that a product must be assessed and conform to “meet high safety, health, and environmental protection requirements” within the European Economic Area.
Health Canada provided approval for the LIGHTSITE1 clinical trial to treat dry Age-related Macular Degeneration (AMD) with the help of a grant from the National Institute of Health (NIH) and the National Eye Institute. The trial has completed its recruitment of 30 patients with dry AMD in July 2018 in Toronto, Canada.
Patients received two rounds of treatment using the LumiThera device. Each three week round consisted of nine sessions. Each session is less than five minutes per eye. The second round of treatment will be administered six months later and patients will be followed for 12 months. Because it is a double blind, randomized, placebo controlled study not all participants will receive the PBM therapy.
"LumiThera has demonstrated promising data with their PBM approach already," says Dr. Robert Devenyi, Ophthalmologist-in-Chief and Director of Retinal Services, University of Toronto. "Recent work showing PBM can improve vision and reduce duress suggests disease-modifying benefits, and that would be very significant for the treatment of dry AMD."
LumiThera LIGHTSITE II
Since the completion of LIGHTSITE I, LumiThera Inc., enrolled patients with dry age-related macular degeneration in LIGHTSITE II in eight retinal centers in Europe including UK, Germany, Spain, Italy and France.
Approximately 100 patients enrolled in the study and will receive treatment for about one year. The purpose of the study will continue to demonstrate safety and according to the LumiThera press release, it will look at "key efficacy endpoints including visual acuity, contrast sensitivity, and reduction of drusen deposits."
This study is no longer recruiting patients and is active but not completed.
Study of Photobiomodulation to Treat Dry Age-Related Macular Degeneration (LIGHTSITE II)
U.S. Multi-Center, LIGHTSITE III Clinical Study
The multi-center U.S. LIGHTSITE III completed enrollment of 100 patients who have dry AMD in February 2021 at one of the participating eye centers, Byers Eye Institute at Stanford University in Palo Alto, CA with Principal Investigator, Diana Do,
M.D. "We are very pleased to be able to enroll the last subject in the
Study! Initial efficacy data will be available in approximately 13
months," indicated Dr. Do. "The study will continue to follow patients
for up to 24 months." There are 11 total participating eye centers including Duke Eye Center in Durham, N.C. and New York Ear and Eye Infirmary in New York, New York.
Patients will be treated and followed for up to 24 months. The purpose of the study involves ..."In addition to demonstrating safety, key efficacy endpoints include visual acuity, contrast sensitivity and reduction of drusen deposits."
Study of Photobiomodulation to Treat Dry Age-Related Macular Degeneration (LIGHTSITE III)
MicroPulse Technology
What makes the MicroPulse laser therapy for macular degeneration different?
Spares the Retina
Unlike hot lasers, the MicroPulse laser cools the tissue between laser pulses. The retinal tissue returns to normal or baseline temperature before the next pulse. This cooling allows for less tissue damage.
Dr. Adams states, "It doesn't burn or damage the retina and the results are often excellent."
Repeatable Sessions
Patients can be retreated when needed.
Safer
Watch this video to get an explanation from Dr. Neal Adams, MD, and board certified ophthalmologist from DC Retina in Silver, Spring, MD.
According to the Iridex website:
"MicroPulse allows the tissue to cool between laser pulses, minimizing or preventing tissue damage. Treatment risks are reduced or eliminated, with increased patient comfort than with conventional, continuous-wave laser treatment."
Go from Laser Therapy for Macular Degeneration to Wet AMD Treatment
Return to WebRN Macular Degeneration Home
√ Prevention of Macular Degeneration?
√ Tips for Daily Living?
√ Food Suggestions for a Macular Degeneration Diet?
√ Ideas on Visual Aids to Maximize your Sight?
If you said "yes" to any of the above, sign up for the monthly Macular Degeneration News.